L-012化学发光探针
生化学用 for Biochemistry
规格含量 : 90.0+%(HPLC)
制造商 : FUJIFILM Wako Pure Chemical Corporation(富士胶片和光)
储存条件:冷藏(干冰运输)
CAS RN® : 143556-24-5
分子式 : C13H8ClN4NaO2
分子量 : 310.67
◆基本信息
L-012 是一种高灵敏度的化学发光(CHL)探针,比鲁米诺(Luminol)试剂更有效。L-012 与多种类型的活性氧反应,这些活性氧来自人血液和口腔中激活的中性粒细胞或者大鼠腹膜腔。该产品可以用于有辣根过氧化物酶的ELISA 试剂盒,可提高反应灵敏度。
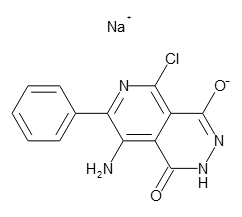
结构式
溶解及保存:蒸馏水溶解成20 mM/L(6.23 mg/mL)的水溶液,再用50 mM/L的Tris盐酸缓冲液(pH 7.5)稀释 至最终浓度为500 μM/L(有细胞的反应体系)。另外,在没细胞参与的反应体系中时,建议使用终浓度为5~50 μM/L。母液冷冻保存,使用时再稀释。
◆物性信息
外观
白色~黄色,结晶~粉末
溶解性
可溶于水。
Q: L-012能渗入细胞吗?
A: 由于L-012对脂质高度亲和,它比luminol和lucigenin更能具有细胞渗透性。另一方面,ROS无限制地在胞外释放。因此L-012被认为也能
检测胞外ROS。
Q: L-012的使用浓度如何?
A: 这取决于应用和条件。
[体外] 培养细胞(EoL-1细胞): 500 ~ 800 μmol/L.
全血和分离的中性粒细胞: 400 μmol/L
多形核中性粒细胞(PMNs)(由小鼠中分离): 2 mmol/L
无细胞的样品: 4 μmol/L, 15 μmol/L, and 50 μmol/L
[体内] Balb/c 小鼠[皮下]: 20 ~ 80 μL of L-012(5mg/mL)
Balb/c 小鼠[腹膜内]: 100 μL of L-012 (15mg/mL)
C57BL/10.Q (B10.Q) 小鼠 [腹膜内]: 1 ~ 75 mg/kg
Q: 是否可用于动物活体成像?
A: 如果所用的小动物成像仪中有发光检测探头,就可以进行检测。以下是文献报道过的仪器型号:
・IVIS 100 imaging systems
・Infinite M200 [Tecan]
・Luminescence Reader BLR-201 [Aloka]
・Beckman-Coulter DTX880 multimode plate reader [Beckman]
Q: 是否可用于固定细胞?
A: 目前尚无证据表明,活性氧(ROS)在细胞活性停止后仍然存在。普遍看法是认为在细胞活性停止后(细胞死亡)ROS消失。固定组织或
细胞会停止细胞活性,因此不建议用于ROS检测。
本品无需固定检测对象即可检测到ROS,使用方法包括但不限于将其添加到细胞培养上清液中或进行动物腹腔注射。
参考文献
|
1. |
Time-series transcriptome of Brachypodium distachyon during bacterial flagellin-induced pattern-triggered immunity Tsubasa Ogasahara, Yusuke Kouzai, Megumi Watanabe, Akihiro Takahashi, Kotaro Takahagi, June-Sik Kim, Hidenori Matsui, Mikihiro Yamamoto, Kazuhiro Toyoda, Yuki Ichinose, Keiichi Mochida, Yoshiteru Noutoshi Front Plant Sci. 2022; 13: 1004184. Published online 2022 Sep 15. doi: 10.3389/fpls.2022.1004184 PMCID: PMC9521188 |
|
2. |
Ruscogenins Improve CD-Like Enteritis by Inhibiting Apoptosis of Intestinal Epithelial Cells and Activating Nrf2/NQO1 Pathway Hexin Wen, Xiaofeng Zhang, Qingqing Li, Ju Huang, Guangyong Liu, Jingyue Zhao, Yiran Liu, Li Shen, Yuyang Li, Kun Yang, Lugen Zuo, Jing Li, Jing Nian, Ping Xiang, Hao Zhao, Liang Yu, Mulin Liu, Zhijun Geng, Xue Song Oxid Med Cell Longev. 2022; 2022: 4877275. Published online 2022 Mar 10. doi: 10.1155/2022/4877275 PMCID: PMC8930266
|
|
3. |
TOPLESS promotes plant immunity by repressing auxin signaling and is targeted by the fungal effector Naked1 Fernando Navarrete, Michelle Gallei, Aleksandra E. Kornienko, Indira Saado, Mamoona Khan, Khong-Sam Chia, Martin A. Darino, Janos Bindics, Armin Djamei Plant Commun. 2022 Mar 14; 3(2): 100269. Published online 2021 Dec 17. doi: 10.1016/j.xplc.2021.100269 PMCID: PMC9073326 |
|
4. |
Noninvasive imaging of the lung NETosis by anti-Ly6G iron oxide nanoparticles Jianghong Zhong, Chanyu Zheng, Haiqiang Gao, Wei Tong, Hui Hui, Jie Tian Heliyon. 2022 Aug; 8(8): e10043. Published online 2022 Aug 3. doi: 10.1016/j.heliyon.2022.e10043 PMCID: PMC9382280 |
|
5. |
Glycolysis and the Pentose Phosphate Pathway Promote LPS-Induced NOX2 Oxidase- and IFN-β-Dependent Inflammation in Macrophages Jonathan R. Erlich, Eunice E. To, Raymond Luong, Felicia Liong, Stella Liong, Osezua Oseghale, Mark A. Miles, Steven Bozinovski, Robert D. Brooks, Ross Vlahos, Stanley Chan, John J. O’Leary, Doug A. Brooks, Stavros Selemidis Antioxidants (Basel) 2022 Aug; 11(8): 1488. Published online 2022 Jul 29. doi: 10.3390/antiox11081488 PMCID: PMC9405479 |
|
6. |
Identification of QTL for Target Leaf Spot resistance in Sorghum bicolor and investigation of relationships between disease resistance and variation in the MAMP response Jennifer Kimball, Yaya Cui, Dongqin Chen, Pat Brown, William L. Rooney, Gary Stacey, Peter J. Balint-Kurti Sci Rep. 2019; 9: 18285. Published online 2019 Dec 4. doi: 10.1038/s41598-019-54802-x PMCID: PMC6893015 |
|
7. |
Chemical genetic identification of a lectin receptor kinase that transduces immune responses and interferes with abscisic acid signaling Jiyoung Park, Tae-Houn Kim, Yohei Takahashi, Rebecca Schwab, Keini Dressano, Aaron B Stephan, Paulo HO Ceciliato, Eduardo Ramirez, Vince Garin, Alisa Huffaker, Julian I Schroeder Plant J. Author manuscript; available in PMC 2020 May 1. Published in final edited form as: Plant J. 2019 May; 98(3): 492–510. Published online 2019 Mar 7. doi: 10.1111/tpj.14232 PMCID: PMC6488365 |
|
8. |
Intranasal and epicutaneous administration of Toll-like receptor 7 (TLR7) agonists provides protection against influenza A virus-induced morbidity in mice Eunice E. To, Jonathan Erlich, Felicia Liong, Raymond Luong, Stella Liong, Steven Bozinovski, Huei Jiunn Seow, John J. O’Leary, Doug A. Brooks, Ross Vlahos, Stavros Selemidis Sci Rep. 2019; 9: 2366. Published online 2019 Feb 20. doi: 10.1038/s41598-019-38864-5 PMCID: PMC6382773 |
|
9. |
Mixed Linkage β-1,3/1,4-Glucan Oligosaccharides Induce Defense Responses in Hordeum vulgare and Arabidopsis thaliana Sina Barghahn, Gregory Arnal, Namrata Jain, Elena Petutschnig, Harry Brumer, Volker Lipka Front Plant Sci. 2021; 12: 682439. Published online 2021 Jun 17. doi: 10.3389/fpls.2021.682439 PMCID: PMC8247929 |
|
10 |
The Pleiades are a cluster of fungal effectors that inhibit host defenses Fernando Navarrete, Nenad Grujic, Alexandra Stirnberg, Indira Saado, David Aleksza, Michelle Gallei, Hazem Adi, André Alcântara, Mamoona Khan, Janos Bindics, Marco Trujillo, Armin Djamei PLoS Pathog. 2021 Jun; 17(6): e1009641. Published online 2021 Jun 24. doi: 10.1371/journal.ppat.1009641 PMCID: PMC8224859
|
|
11. |
Genome-wide association analysis of the strength of the MAMP-elicited defense response and resistance to target leaf spot in sorghum Rozalynne Samira, Jennifer A. Kimball, Luis Fernando Samayoa, James B. Holland, Tiffany M. Jamann, Patrick J. Brown, Gary Stacey, Peter J. Balint-Kurti Sci Rep. 2020; 10: 20817. Published online 2020 Nov 30. doi: 10.1038/s41598-020-77684-w PMCID: PMC7704633 |
|
12. |
Engineering Smut Resistance in Maize by Site-Directed Mutagenesis of LIPOXYGENASE 3 Krishna Mohan Pathi, Philipp Rink, Nagaveni Budhagatapalli, Ruben Betz, Indira Saado, Stefan Hiekel, Martin Becker, Armin Djamei, Jochen Kumlehn Front Plant Sci. 2020; 11: 543895. Published online 2020 Oct 21. doi: 10.3389/fpls.2020.543895 PMCID: PMC7609844 |
|
13. |
Rapid repeatable in vivo detection of retinal reactive oxygen species Ning Fan, Sean M. Silverman, Yang Liu, Xizhen Wang, Byung-Jin Kim, Liping Tang, Abbot F. Clark, Xuyang Liu, Iok-Hou Pang Exp Eye Res. Author manuscript; available in PMC 2018 Aug 1. Published in final edited form as: Exp Eye Res. 2017 Aug; 161: 71–81. Published online 2017 Jun 8. doi: 10.1016/j.exer.2017.06.004 PMCID: PMC5554724 |
|
14. |
A rapid bioluminescence assay for measuring myeloperoxidase activity in human plasma Reece J. Goiffon, Sara C. Martinez, David Piwnica-Worms Nat Commun. 2015 Feb 10; 6: 6271. Published online 2015 Feb 10. doi: 10.1038/ncomms7271 PMCID: PMC4347050 |
|
15. |
CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity Kim-Kristin Prior, Matthias S. Leisegang, Ivana Josipovic, Oliver Löwe, Ajay M. Shah, Norbert Weissmann, Katrin Schröder, Ralf P. Brandes Redox Biol. 2016 Oct; 9: 287–295. Published online 2016 Aug 24. doi: 10.1016/j.redox.2016.08.013 PMCID: PMC5021817 |