StemSure® hPSC MediumΔ
This product is a serum-free liquid media suitable for maintaining human pluripotent stem cells (hPSCs), human ES cells and human iPS cels in the absence of feeder cells. The raw material of this media is not used components containing any animal-derived materials. Moreover, this media does not contain any albumins (such as BSA or HSA), thereby it can culture hPSCs stably without lot difference. Please note that this medium does not contain basic fibroblast growth factor (bFGF).
- Features
- Quality Control Testing
- Usage Notes
- Culture of human iPS Cells 201B7 Strain
- Culture of human ES Cells WA01 Strain
- Karyotyping
- Differentiation of Human iPS 201B7 Cells into Three Germ Layers
- Product List
- Related Information
Features
- Feeder-free, serum-free media for culturing hPSCs.
- Quality control testing of each lot is performed using the human iPS cell 201B7 strain.
- Animal component-free.
- Albumin-free, low-protein media.
- Compatible with various culture vessel surface coating reagents, such as Matrigel®, iMatrix-511 and vitronectin.
- Compatible with various cell dissociation reagents, such as Accutase and TrypLE Select.
- Enables single-cell passaging by adding Y-27632 when passage.
Quality Control Testing
- Cell proliferation assay (using the human iPS cell 201B7 strain)
- Alkaline phosphatase staining (using the human iPS cell 201B7 strain)
- Sterility test
- pH
- Osmolality
- Endotoxin test
- Mycoplasma negative test
Usage Notes
This product does not contain basic fibroblast growth factor (bFGF).
Store frozen at –20°C. After thawed, store at 2-10°C for up to one week. Do not refreeze.
Culture of human iPS Cells 201B7 Strain
Human iPS cells 201B7 strain were first cultured in BSA-containing, feeder-free media manufactured by Competitor A. Subsequently, these cells (passage 0) were transferred to StemSure® hPSC MediumΔ (passage 1) and cultured successively. During subculture, we analyzed their morphology, population doubling levels, and undifferentiated state. The results are shown below.
-
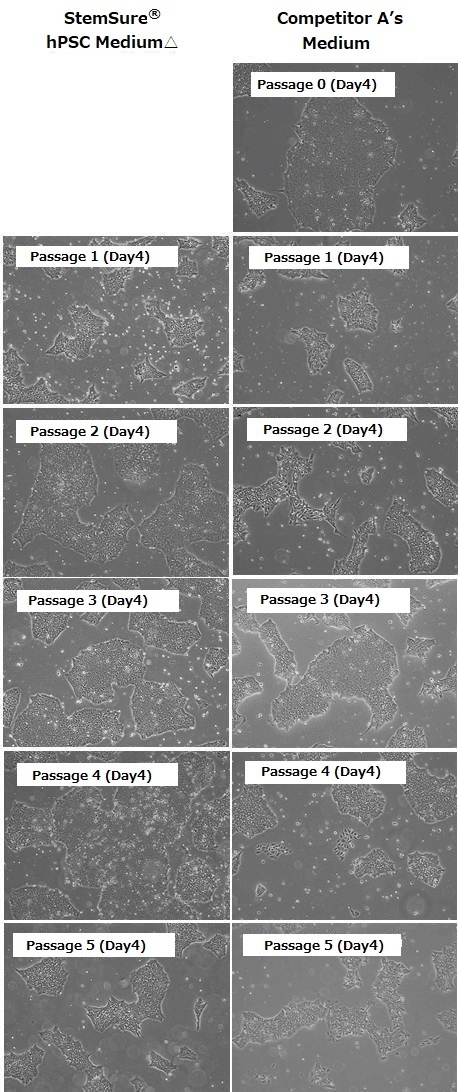
Cell Morphology
Human iPS cells 201B7 strain were first cultured in BSA-containing, feeder-free media manufactured by Competitor A. Subsequently, these cells (passage 0) were transferred to StemSure hPSC MediumΔ (passage 1) and cultured successively. During subculture, we analyzed their morphology, population doubling levels, and undifferentiated state. The results are shown below.

-
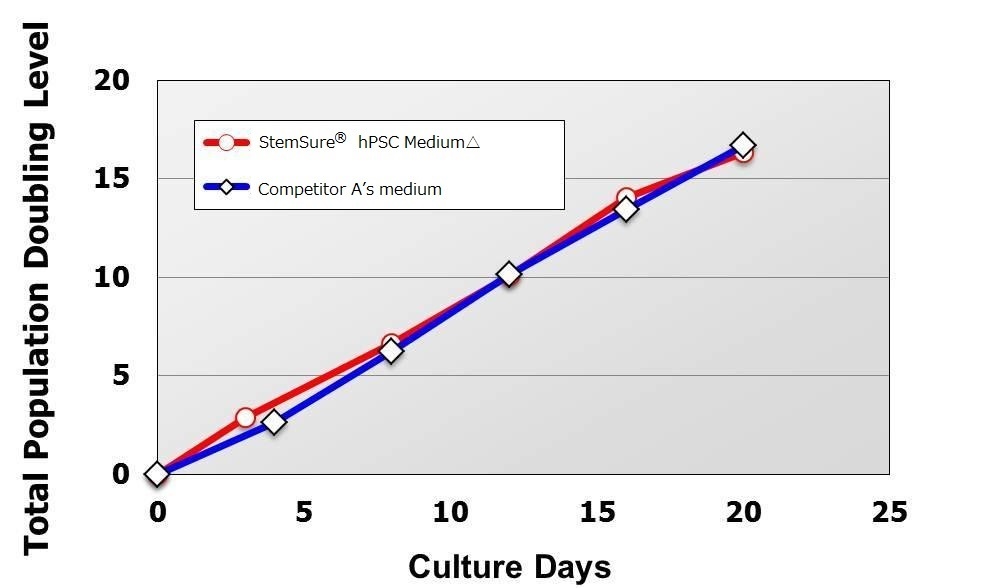
Population Doubling Level
Compared with cells maintained in Competitor A’s medium, human iPS cells transferred to StemSure® hPSC Medium Δ grew faster during the culture period ranging from passage 1 (at the time of cell transfer) to passage 5, and even after.

< Media Compsition >
StemSure® hPSC MediumΔ + 35ng/ml bFGF
< Seeding Density >
1×105cells/well(cultured in 6-well plate)
Maintenance of Undifferentiated State
Cultured in StemSure® hPSC MediumΔ (passage 5) and checked expression of the undifferentiated markers (Nanog, Oct3/4, Tra-1-60, SSEA-4, and BC2LCN).
※BC2LCN is a recombinant lectin with high affinity for glycans that exists on the surface of hPSCs.
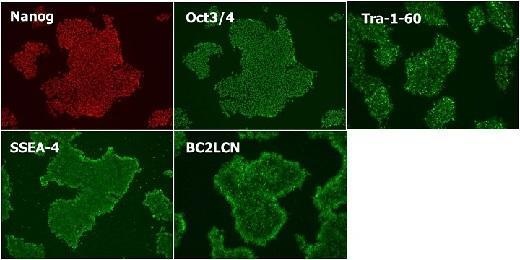
<Data provided by Dr. Yasuko Onuma and Dr. Yuzuru Ito of the Research Center for Stem Cell Engineering at the National Institute of Advanced Industrial Science and Technology.>
Culture of human ES Cells WA01 Strain
Human ES Cells WA01 strain were first cultured in BSA-containing, feeder-free media manufactured by Competitor A. Subsequently, these cells (passage 0) were transferred to StemSure hPSC Medium (passage 1) and cultured successively. During subculture, we analyzed their morphology, population doubling levels, and undifferentiated state. The results are shown below.
-
Cell Morphology
When cultured in Competitor A’s media, cells of differentiated morphology appeared after passage 2. In contrast, human ES cells cultured in StemSure® hPSC Medium Δ did not show any morphological changes. This indicates that StemSure hPSC MediumΔ can maintain the undifferentiated state of human ES cells.
-
Population Doubling Level
There was no difference in cell proliferation ability compared to the Competitor A’ media from passage 1 after transfer to StemSure® hPSC MediumΔ until passage 5.
< Media Composition >
StemSure® hPSC MediumΔ + 35ng/ml bFGF
< Seeding Density >
1×105cells/well(cultured in 6-well plate)
Maintenance of Undifferentiated State
Cultured in StemSure® hPSC MediumΔ (passage 6) and checked expression of the undifferentiated markers(Nanog, Oct3/4, Tra-1-60, SSEA-4, and BC2LCN).
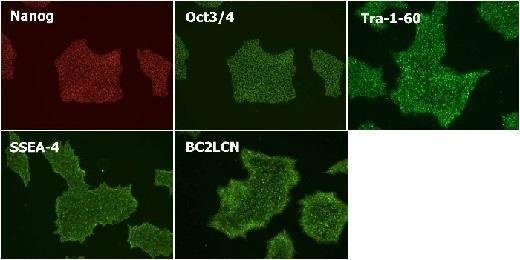
< Data provided by Dr. Yasuko Onuma and Dr. Yuzuru Ito of the Research Center for Stem Cell Engineering at the National Institute of Advanced Industrial Science and Technology. >
Karyotyping
Karyotype analysis revealed no chromosomal abnormalities in human iPS cells passaged 27 times in StemSure® hPSC MediumΔ.
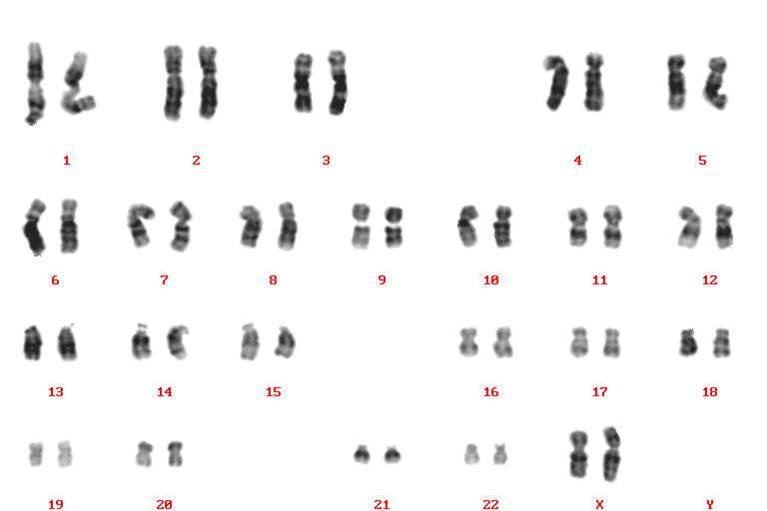
< Data provided by Dr. Takumi Miura and Dr, Hidenori Akutsu of the Center for Regenerative Medicine at the National Research Institute for Child Health and Development. >
Differentiation of Human iPS 201B7 Cells into Three Germ Layers
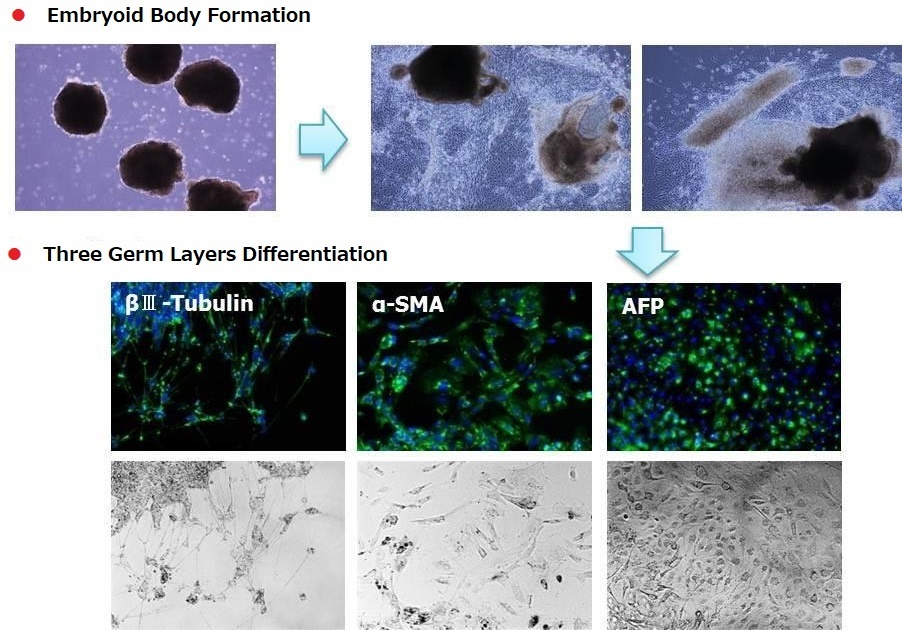
< Media Composition >
StemSure® D-MEM + StemSure® Serum
Replacement + 2mmol/l L-Glutamine +
0.1mmol/l StemSure® 2-Mercaptoetanol + 1
x Non-essential Amino Acids Solution
Product List
- Open All
- Close All
Basic fibroblast growth factor, Y-27632
StemSure® series
Related Information
Category
- Cell Culture
- Stem Cell Culture
- Medium
- Small Molecules for ES/iPS cells Research
- CultureSure® Small Molecules
- MF-registered Small Molecules
- Fluorescent-labeled rBC2LCN
Product content may differ from the actual image due to minor specification changes etc.